 For many cancer patients, clinical trials offer a way to change the course of cancer research--and their own disease.
For many cancer patients, clinical trials offer a way to change the course of cancer research--and their own disease.
For many cancer patients, hope can come from the unknown. Clinical trials for untested drugs can offer those who have not responded to conventional treatments a role in cancer research and a chance at recovery.
Clinical trials usually progress through four phases. Phase one, with 20 to 80 participants, determines safety and dosage range while identifying side effects. Phase two, given to a larger group, tests effectiveness and safety.
Phase three participants, who can number in the thousands, continue to provide data on effectiveness and safety. Their results are compared to commonly used treatments for the same disease. Phase four, where even more data is gathered, occurs after a drug is approved by the government.
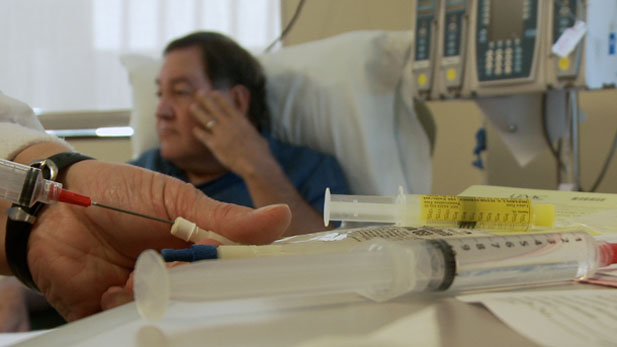
Throughout the process, selecting appropriate trial participants is critical. Andy, who receives his Friday treatments at the University of Arizona Cancer Center, is one of them.
Andy is being treated for GIST, short for gastrointestinal stromal tumors. Over the last decade, he has been treated with tumor resections and with a medication that recently stopped working for him. There is only one other drug on the market that is effective on this type of cancer, so Andy is taking a chance on the drugs of the future.

By submitting your comments, you hereby give AZPM the right to post your comments and potentially use them in any other form of media operated by this institution.